Abstract
Lung transplant recipients receive lifelong immunosuppression that includes a calcineurin inhibitor (tacrolimus or cyclosporine), an anti-proliferative agent (mycophenolate or azathioprine) and corticosteroids. They are also known to be at increased risk for a broad spectrum of adverse hematologic events. This includes relatively common complications such as neutropenia and other cytopenias, which occur in over half of patients, as well as rarer events such as de novo hematopoietic malignancy. Certain patterns of clonal hematopoiesis, the expansion of unique somatic clones in hematopoietic stem cells, are known to be associated with cytotoxic therapy and the development of hematologic malignancies. We hypothesized that the stress of transplantation and subsequent immunosuppression provides an environment whereby a unique pattern of clonal hematopoiesis emerges.
To evaluate this hypothesis, we designed a custom panel of 59 genes using an error-corrected sequencing assay capable of detecting variant allele frequency (VAF) as low as 0.01. We characterized the overall burden and distribution of clonal hematopoiesis and compared differences among lung transplant recipients (n=73) and age-matched healthy controls (n=19). Clonal hematopoiesis was identified in 51/73 (69.9%) of lung transplant recipients. This was significantly higher than the frequency in the age-matched healthy controls (6/19,31.6% p=0.0071). Additionally, 27/51 (52.9%) of patients with clonal hematopoiesis had multiple variants. The increase in clonal hematopoiesis was mainly due to mutations in DNA damage response (DDR) genes (ATM, PPM1D, SRCAP or TP53), with 29/73 (39.7%) of lung transplant recipients carrying one or more mutation compared with 1/19 (5.3%) of age-matched healthy controls (5.3%, p=0.029). No significant difference in the frequency of clonal hematopoiesis due to non-DDR genes was also observed (48% vs. 26.3%, p=0.121).
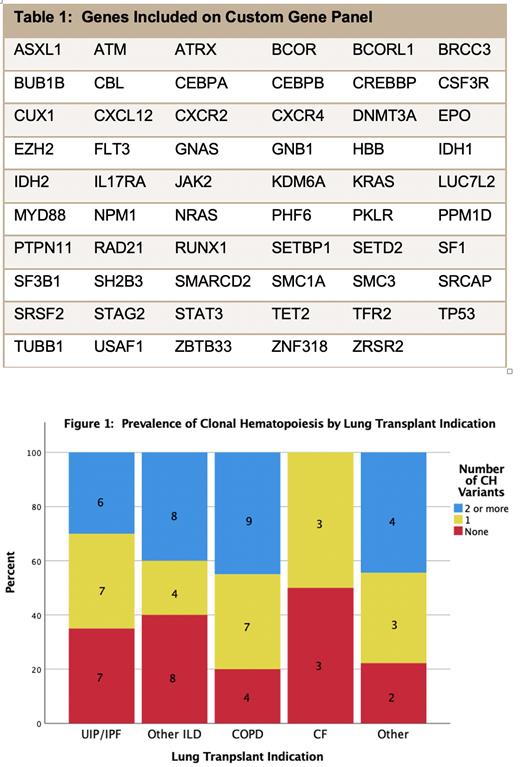
We first evaluated the relationship between clonal hematopoiesis and lung transplant indication. This is relevant, since interstitial lung disease (ILD), a common indication for lung transplantation, is associated with telomeropathies which are also linked to bone marrow failure and clonal hematopoiesis. However, the frequency of DDR clonal hematopoiesis in ILD patients (17/39, 44%) was similar to that observed in patients with COPD (9/20, 45%). We next investigated the association of immunosuppression with clonal hematopoiesis. Overall, 30/48 patients on mycophenolate (MPA) and 13/19 patients on azathioprine (AZA) had at least 1 clonal hematopoiesis mutation. We found no significant difference in overall clonal hematopoiesis frequency among patients on MPA vs. AZA. However, the frequency of DDR clonal hematopoiesis was significantly higher in patients on AZA (OR 4.04, 95% CI 1.22-13.38, p=0.022) than in patients on MPA. Moreover, when we assessed clonal hematopoiesis burden via Poisson regression, it was increased in patients receiving AZA (1.68, 95% CI 1.08-2.59, p=0.020) when compared to patients on MPA. All lung transplant recipients were maintained on tacrolimus but one, so associations with type of calcineurin inhibitor could not be assessed. Finally, we assessed clonal hematopoiesis in recipients who developed neutropenia (n=24) and found no significant association.
To the best of our knowledge, we report the first evidence of increased clonal hematopoiesis in a solid organ transplant population. Our data indicate that azathioprine therapy is associated with the expansion of hematopoietic clones carrying variants in DDR genes. However, azathioprine therapy often represents a failure of MPA therapy, typically due to either hematologic or gastrointestinal toxicity. Therefore, the association noted might reflect MPA intolerance rather than azathioprine therapy. Nevertheless, prior studies show that treatment with genotoxic agents, such as chemotherapy or radiation therapy, provide a fitness advantage to hematopoietic stem/progenitor cells carrying DDR gene variants. Whether azathioprine vs. MPA therapy confers a similar fitness advantage is currently under investigation. Further investigation also is warranted to determine if the presence of clonal hematopoiesis influences lung transplant outcomes.
No relevant conflicts of interest to declare.